発表論文 (Publications)
学術論文(publications)
2025年 | 2024年 | 2023年 | 2022年 | 2021年 | 2020年 | 2019年 | 2018年 | 2017年 | 2016年 | 2015年 | 2014年 | 2013年 | 2012年 | 2011年 | 2010年 | 2009年 | 2008年 | 2007年 | 2006年 | 2005年 | 2004年
2016
- Perfluoroalkyl Analogues of Diethylaminosulfur Trifluoride: Reagents for Perfluoroalkylthiolation of Active Methylene Compounds under Mild Conditions
Saidalimu, I.; Suzuki, S.; Yoshioka, T.; Tokunaga, E.; Shibata, N.*
Org. Lett. 2016, 18, 6404-6407. [Abstract]
DOI: 10.1021/acs.orglett.6b03301
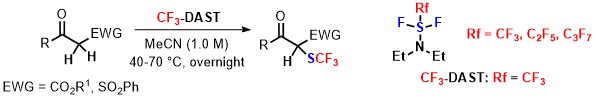
- Alkynyl Cinchona Catalysts affect Enantioselective Trifluoromethylation for Efavirenz under Metal-Free Conditions
Okusu, S.; Hirano, K.; Yasuda, Y.; Tanaka, J.; Tokunaga, E.; Fukaya, H.; Shibata, N.*
Org. Lett. 2016, 18, 5568-5571. [Abstract]
DOI: 10.1021/acs.orglett.6b03301
Synfact誌に紹介されました。Synfacts 2017; 13(01): 0004, DOI: 10.1055/s-0036-1589743
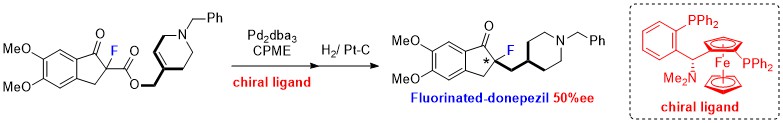
- Synthesis of fluorinated donepezil by palladium-catalyzed decarboxylative allylation of α-fluoro-β-keto ester with tri-substituted heterocyclic alkene and the self-disproportionation of its enantiomers
Maeno, M.; Kondo, H.; Tokunaga, E.; Shibata, N.*
RSC Adv. 2016, 6, 85058-85062. [Abstract]
DOI: 10.1039/C6RA21253K
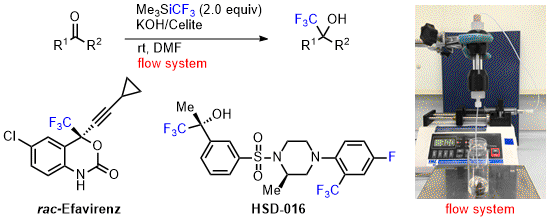
- Flow trifluoromethylation of carbonyl compounds by Ruppert-Prakash reagent and its application for pharmaceuticals, Efavirenz and HSD-016
Okusu, S.; Hirano, K.; Yasuda, Y.; Tokunaga, E.; Shibata, N.*
RSC Adv. 2016, 6, 82716-82720. [Abstract]
DOI: 10.1039/C6RA19790F
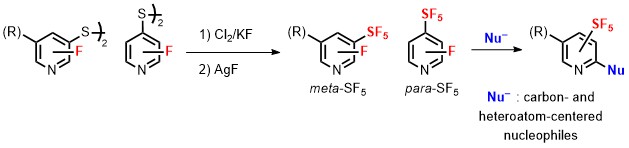
- Importance of a Fluorine Substituent for the Preparation of meta- and para-Pentafluoro-λ6-sulfanyl-Substituted Pyridines
Kosobokov, M.; Cui, B.; Balia, A.; Matsuzaki, K.; Tokunaga, E.; Saito, N.; Shibata, N.*
Angew. Chem., Int. Ed. 2016, 55, 10781-10785. [Abstract]
DOI: 10.1002/anie.201605008
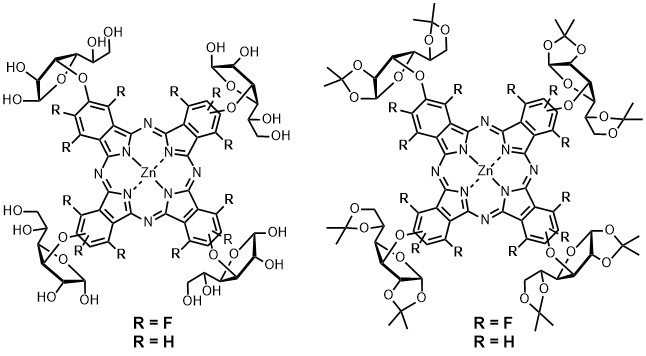
- Synthesis and Optical Properties of Fluorine-Containing Phthalocyanine Conjugated with Glucofuranose and its Application to Photo-Dynamic Therapy
Mori, S.; Tokunaga, E.; Hayashi, M.; Obata, T.; Tanaka, M.; Shibata, N.*
J. Jpn. Soc. Colour Mater. 2016, 89, 213-218. [Abstract] J-Stage]
DOI: 10.4011/shikizai.89.213
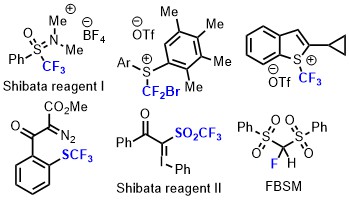
- Development of Shelf-Stable Reagents for Fluoro-Functionalization Reactions
Shibata, N.
Bull. Chem. Soc. Jpn. 2016, 89, 1307-1320. [Abstract]
DOI: 10.1246/bcsj.20160223
- Assessment of Protein Binding of 5-Hydroxythalidomide Bioactivated in Humanized Mice with Human P450 3A-Chromosome or Hepatocytes by Two-Dimensional Electrophoresis/Accelerator Mass Spectrometry
Yamazaki, H.*; Suemizu, H.; Kazuki, Y.; Oofusa, K.; Kuribayashi, S.; Shimizu, M.; Ninomiya, S.; Horie, T.; Shibata, N.; Guengerich, F. P.
Chem. Res. Toxicol. 2016, 29, 1279-1281. [Abstract]
DOI: 10.1021/acs.chemrestox.6b00210
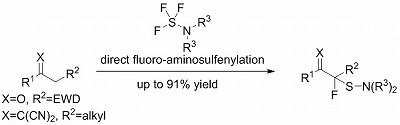
- Direct Fluoro-aminosulfenylation of Active Methylenes by Dialkylaminosulfur Trifluorides under Catalyst-Free Conditions
Saidalimu, I.; Guo, M.; Tokunaga, E.; Shibata, N.*
Asian J. Org. Chem. 2016, 5, 1208-1212. [Abstract] (Cover Picture)
DOI: 10.1002/ajoc.201600264
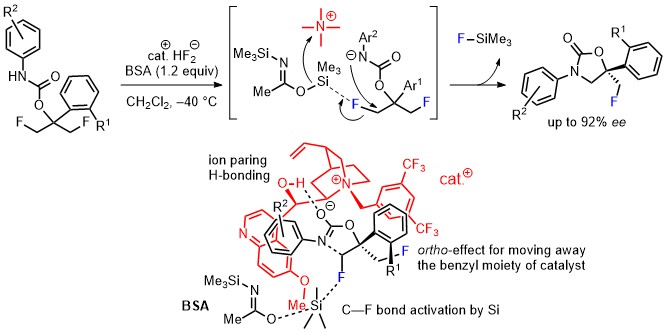
- Asymmetric Desymmetrization via Metal-Free C-F Bond Activation: Synthesis of 3,5-Diaryl-5-fluoromethyloxazolidin-2-ones with Quaternary Carbon Centers
Tanaka, J.; Suzuki, S.; Tokunaga, E.; Haufe, G.; Shibata, N.*
Angew. Chem., Int. Ed. 2016, 55, 9432-9436. [Abstract]
DOI: 10.1002/anie.201603210
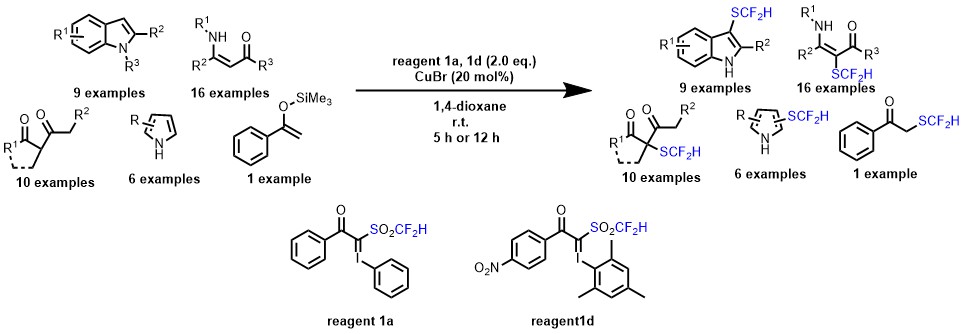
- Difluoromethanesulfonyl hypervalent iodonium ylides for electrophilic difluoromethylthiolation reactions under copper catalysis
Arimori, S.; Matsubara, O.; Takada, M.; Shiro, M.; Shibata, N.*
R. Soc. Open Sci. 2016, 3: 160102. [Abstract]
DOI: 10.1098/rsos.160102
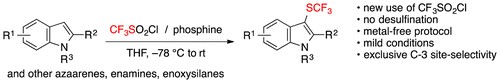
- Novel Use of CF3SO2Cl for the Metal-Free Electrophilic Trifluoromethylthiolation
Chachignon, H.; Maeno, M.; Kondo, H.; Shibata, N.*; Cahard, D.*
Org. Lett. 2016, 18, 2467-2470. [Abstract]
DOI: 10.1021/acs.orglett.6b01026
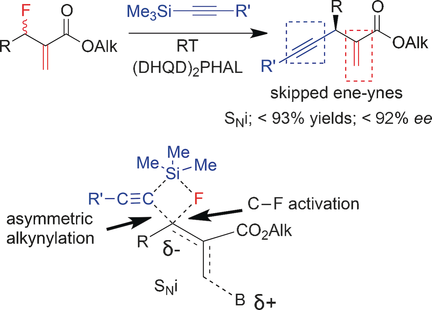
- Organocatalytic Enantioselective Nucleophilic Alkynylation of Allyl Fluorides Affording Chiral Skipped Ene-ynes
Okusu, S.; Okazaki, H.; Tokunaga, E.; Soloshonok, V. A.; Shibata, N.*
Angew. Chem., Int. Ed. 2016, 55, 6744-6748. [Abstract]
DOI: 10.1002/anie.201601928
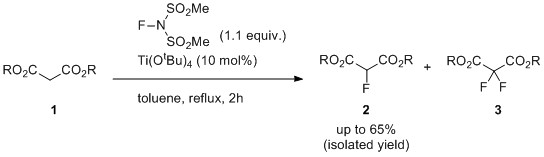
- Lewis Acid-Catalyzed Selective Mono-fluorination of Malonates Using Me-NFSI
Fukushi, K.; Tokunaga, E.; Sumii, Y.; Kagawa, T.; Nagasaki, N.; Shibata, N.*
Fluorine notes 2016, 105. 3-4. [Abstract]
DOI: 10.17677/fn20714807.2016.02.02
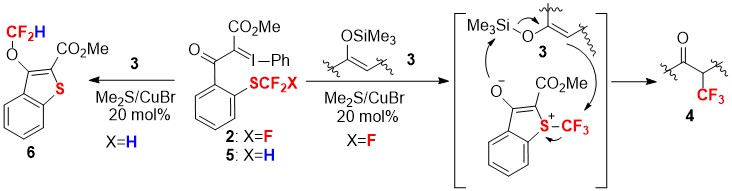
- Activation of Trifluoromethylthio Moiety by Appending Iodonium Ylide under Copper Catalysis for Electrophilic Trifluoromethylation Reaction
Saidalimu, I.; Suzuki, S.; Tokunaga, E.; Shibata, N.*
Chin. J. Chem. 2016, 34, 485-489. [Abstract]
DOI: 10.1002/cjoc.201600029
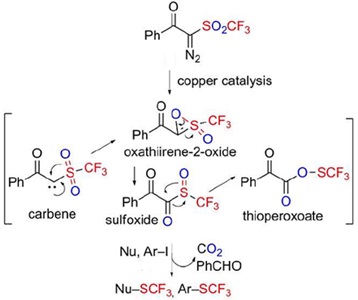
- 2-Diazo-1-phenyl-2-((trifluoromethyl)sulfonyl)ethan-1-one: Another Utility for Electrophilic Trifluoromethylthiolation Reactions
Huang, Z.; Okuyama, K.; Wang, C.; Tokunaga, E.; Li, X.; Shibata, N.*
ChemistryOpen 2016, 5, 188-191. [Abstract]
DOI: 10.1002/open.201500225
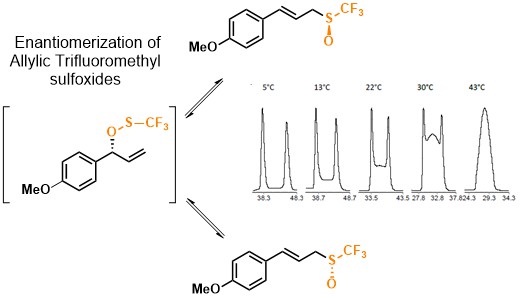
- Enantiomerization of Allylic Trifluoromethyl Sulfoxides Studied by HPLC Analysis and DFT Calculations
Bailly, L.; Petit, E.; Maeno, M.; Shibata, N.; Trapp, O.; Cardinael, P.; Chataigner, I.; Cahard, D.*
Chirality 2016, 28, 136-142. [Abstract]
DOI: 10.1002/chir.22552
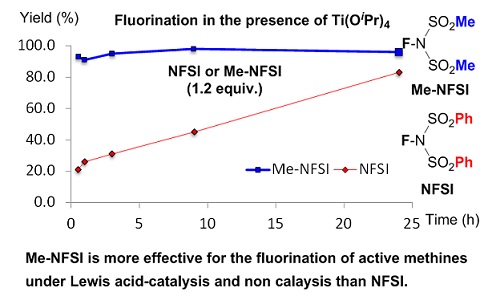
- Methyl NFSI: Atom-economical alternative to NFSI shows higher fluorination reactivity under Lewis acid-catalysis and non-catalysis
Fukushi, K.; Suzuki, S.; Kamo, T.; Tokunaga, E.; Sumii, Y.; Kagawa, T.; Kawada, K.; Shibata, N.*
Green Chem. 2016, 18, 1864-1868. [Abstract]
DOI: 10.1039/C5GC02612A
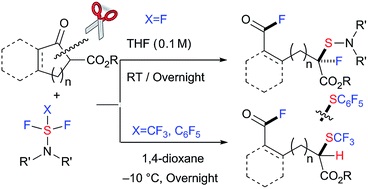
- Successive C-C bond cleavage, fluorination, trifluoromethylthio- or pentafluorophenylthiolation under metal-free conditions to provide compounds with dual fluoro-functionalization
Saidalimu, I.; Suzuki, S.; Tokunaga, E.; Shibata, N.*
Chem. Sci. 2016, 7, 2106-2110. [Abstract]
DOI: 10.1039/C5SC04208A
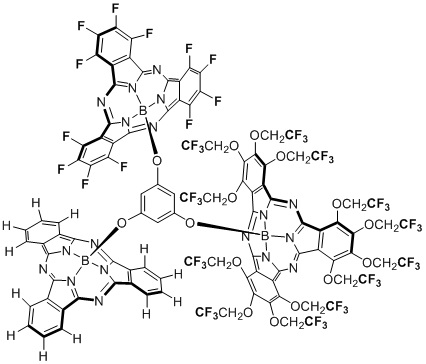
- Design, synthesis and optical properties of unsymmetrical subphthalocyanine trimer connected by phloroglucinol via axial positions
Mori, S.; Ogawa, N.; Tokunaga, E.; Tsuzuki, S.; Shibata, N.*
Dalton Trans. 2016, 45, 908-912. [Abstract]
DOI: 10.1039/C5DT04500B
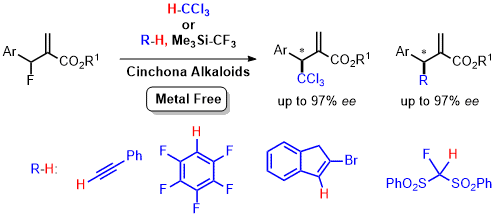
- Enantioselective Trichloromethylation of MBH-Fluorides with Chloroform Based on Silicon-assisted C-F Activation and Carbanion Exchange Induced by Ruppert-Prakash Reagent
Nishimine, T.; Taira, H.; Tokunaga, E.; Shiro, M.; Shibata, N.*
Angew. Chem., Int. Ed. 2016, 55, 359-363. [Abstract]
DOI: 10.1002/anie.201508574