Anesthesia Phenomenon
The rapid progress of modern clinical medicine raises the importance of anesthetics. It is urgently necessary to elucidate
anesthesia mechanisms. Numerous studies of anesthesia have been performed to clarify the origin of anesthesia phenomena since Meyer
and Overton presented the 'lipoid hypothesis' that volatile anesthetics dissolve in hydrophobic lipids parts [1-3]. The latest
trends of general anesthesia theory show two directions: 'lipid theory' and 'protein theory'. Lipid theory holds that anesthetics
interact nonspecifically with the lipid bilayer region of the nerve cell (neuron) and perturb overall membrane functions including
those of membrane proteins. Protein theory holds that anesthetic molecules act directly with nonpolar sites on excitable proteins.
Although more than 150 years have passed since anesthetics were discovered, details of anesthesia mechanisms remain unclear: no
consensus exists.
Anesthetics possess unique characteristics that differ from those of general therapeutic drugs. Anesthesia phenomena occur at
high body concentrations (millimolar-order level), and the effects show reversibility depending on the medicated concentration [4,5].
General therapeutic drugs, on the other hand, act on specific receptor sites at quite low body concentrations (nanomolar-order level),
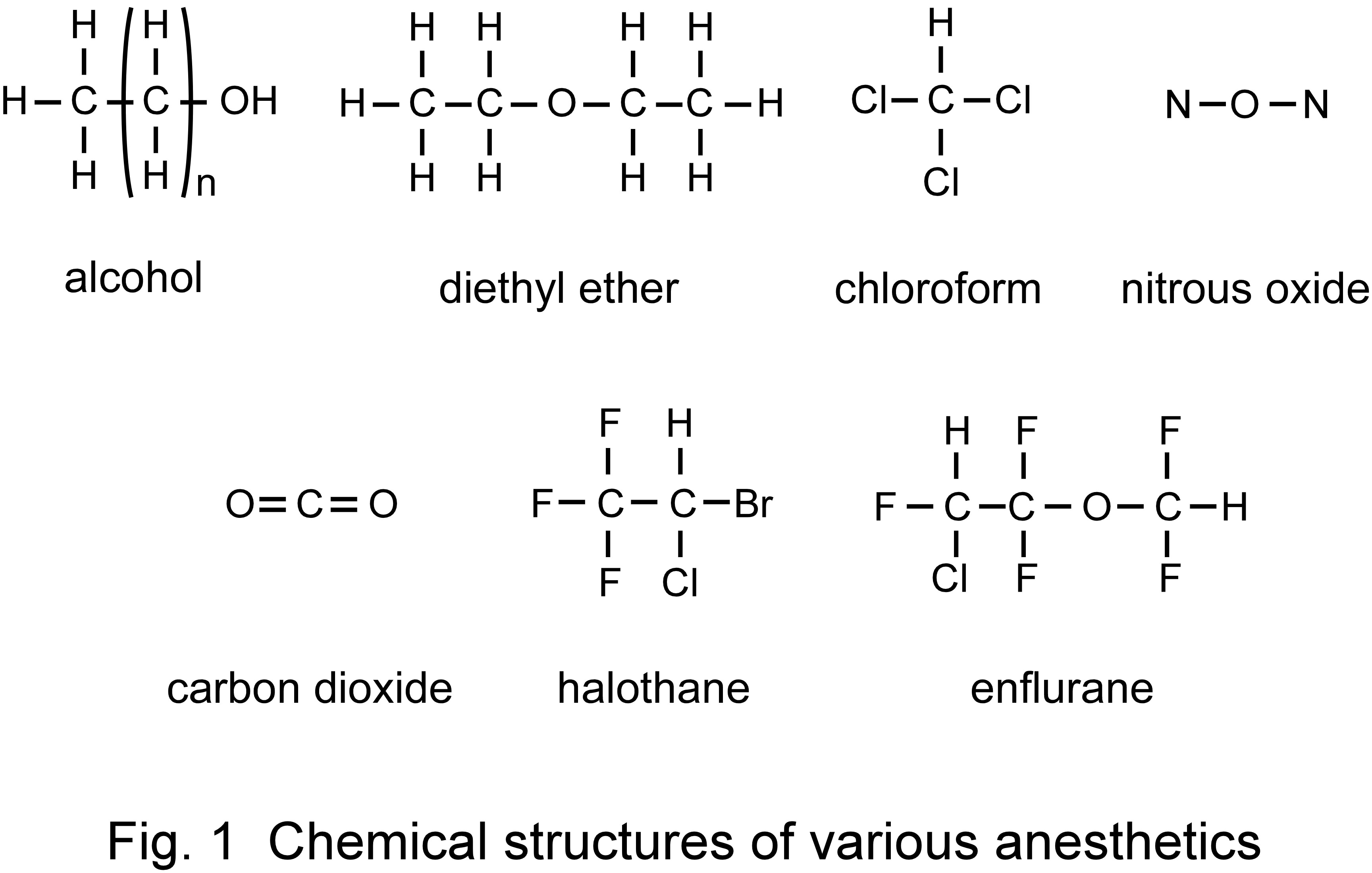
and the effects are irreversible. Anesthetics do not possess specific molecular frameworks (e.g., alcohol, diethyl ether, chloroform,
dinitrogen monoxide, carbon dioxide, halothane, enflurane; Fig. 1). A common characteristic of these anesthetics is that they possess
weak dipole moments and form hydration clusters in which water molecules surround anesthetics [6]. Anesthetic potency (power of
anesthetic action) is also known to show temperature dependence. The anesthetic potencies of diethyl ether and alcohol are strong at
high temperatures, whereas those of halothane and enflurane are strong at low temperatures [7-9]. From these characteristics, the
relation between anesthetics aggregation and the biomembrane/body-fluid interface is related closely to anesthesia phenomenon; anesthesia
would be regarded as 'physisorption phenomenon' by which the anesthetics aggregation acts indirectly on the biomembrane/body-fluid
interface [6,7].
We are investigating the action mechanism of volatile anesthetics on various phospholipid monolayers formed on an air/water interface from the viewpoint of its
effect through anesthetics aggregation and properties of the interfacial layer. The measurement methods are quartz crystal microbalance
(QCM), quartz crystal impedance (QCI), nuclear magnetic resonance (NMR), and interfacial lateral electrical conductivity (ILEC).
References
[1] S. Shimabayashi, H. Terada, H. Okabayashi, Seitai Colloid, Hirokawa Pub. Co., 1990, p. 545.
[2] H. Meyer, Exp. Pathol. Pharmakol. 42 (1899) 109.
[3] E. Overton, Verlag von Gustav Fischer, Jena., 1901.
[4] I. Ueda, T. Yoshida, Chem. Phys. Lipids 101 (1999) 65.
[5] I. Ueda, T. Yoshida, Encyclopedia of Surface and Colloid Science, Dekker, New York, 2002, p. 2607.
[6] T. Yoshida, H. Okabayashi, H. Kamaya, I. Ueda, Biochim. Biophys. Acta 979 (1989) 287.
[7] A. Cherkin, J. F. Catchpool, Science 144 (1964) 1460.
[8] V. Flook, G. D. Adey, C. R. Dundas, D. C. White, J. Appl. Physiol. 37 (1974) 552.
[9] J. D. McKenzie, P. Calow, J. Clyde, A. Miles, R. Dickinson, W. R. Lieb, N. P. Franks, Comp. Biochem. Physiol 101C (1992) 15.
Return to Theme and Contents

